 Hey Reader,
Hey Reader, What is that weird word ion the title: Bohr? Well, here we are talking about the physicist, Neils Bohr, the man responsible for the High School model of the atom! You know, that model that mimics the solar system. How did it come about? Why is it useful? Why is it used?
To start, lets see the history of the atom and the models. It started with Dalton, when he stated that the atom is the smallest unit of matter. He also stated that the atom is just a solid ball (such as a billiard ball). This formed the first theory of the atom
.
Then came J.J. Thompson, who realized that a charge can be induced onto the atom. Negative or positive, there has to be a way to charge an atom. He devised a model (sometimes called the plum-pudding model) in which allows the charges of the atom to be spread-out in a "solid" atom.
50 years later, a famous experiment, called the Gold Foil experiment was performed by Ernest Rutherford. His discover shocked the world of science. He shot positive particles (alpha particles) through a thin gold foil and placed detectors at different angles. He observed that many alpha particles was deflected at very small angles. they almost
passed right through!. How could this be unless there was a central nucleus with a positive charge. Thus, we learned that there was a central positive nucleus.
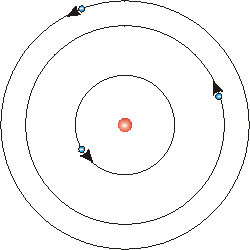
Borh showed that electrons are in orbits. Shown above, electrons circle the nucleus like planets around the sun. The first orbit holds 2 electrons, the 2nd and 3rd each have 8. This is NOT what the current model represents, but this is widely used in many people's education.

This model is useful because it explains a weird phenomenon
. Certain elements emit a certain spectra of light. To the right is a small spectrum (known as the Balmer) that is emitted from Hydrogen. Hydrogen emit other visible lights as well. How can this be explained unless electrons can get excited to different orbitals and de-excited states to lower orbitals. To produce light, with this model, an electron must go from a higher orbital to a lower orbital (Blamer series goes from any higher orbital to the 2nd orbital). This can be mathematically explained using the Rydberg-Balmer equation. The wavelength is correlated to the difference of squared orbitals.
Even though a more accurate model has been discovered, called Valence Shell Electron Pair Repulsion (VSEPR) theory, Borh's model is a very basic model that works well with the spectral phenomenon. We use it so we can better understand the light.